Plutonium At Room Temperature

913 k 640 c or 1184 f boiling point.
Plutonium at room temperature. 3501 k 3228 c or 5842 f density. At room temperature alpha form plutonium the most common form is as hard and brittle as cast iron. What state of matter is plutonium at room temperature. Plutonium atoms have 94 electrons and 94 protons with 2 valence electrons in the outer shell.
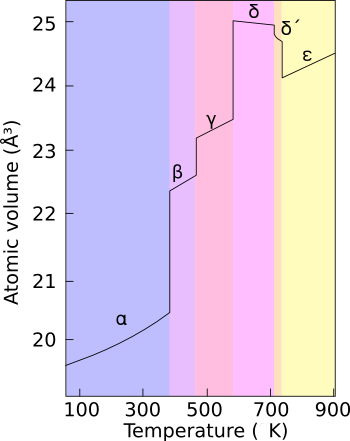
Spontaneously ignites at. The radioactivity of the burning material is an additional hazard. The delta phase has more typical metallic character and is roughly as strong. Unlike most metals it is not a good conductor of heat or electricity.
Plutonium in the δ phase delta phase normally exists in the 310 c to 452 c range but is stable at room temperature when alloyed with a small percentage of gallium aluminium or cerium enhancing workability and allowing it to be welded in weapons applications. It can be alloyed with other metals to form the room temperature stabilized delta form which. It is as hard and brittle as cast iron unless alloyed with other metals to form the room temperature stabilized delta δ phase which makes it soft and ductile. At room temperature plutonium is a solid metal.
Non divided metal at room temperature corrodes relatively inert slowly oxidizes. At room temperature alpha form plutonium is as hard and brittle as cast iron. 19 84 grams per cubic centimeter. 2009 06 14 21 11 27 2009 06 14 21 11 27.
At room temperature for instance the plutonium is very brittle but heated to around 100 celsius is transforms to a much more malleable metal. Scientists have found that they can mimic this effect by adding a small amount of gallium which gives the room temperature metal similar properties to its higher temperature counterpart and this. Phase at room temperature. There are 150 neutrons in the most abundant isotope.
Plutonium expands up to 70 in volume as it oxidizes and thus may break its container. At room temperature plutonium is in its alpha α form the most common structural form of the element. In a moist environment plutonium forms hydrides on its surface which are pyrophoric and may ignite in air at room temperature. Plutonium is a member of the actinide group in the periodic table.
Finely divided particles under about 1 millimeter diameter spontaneously ignites at about 150 c 3 finely particles over about 1 millimeter diameter. Plutonium normally has six allotropes and forms a seventh zeta ζ at high temperature within a limited pressure range. Characteristics and properties under standard conditions plutonium is a hard brittle silvery metal.